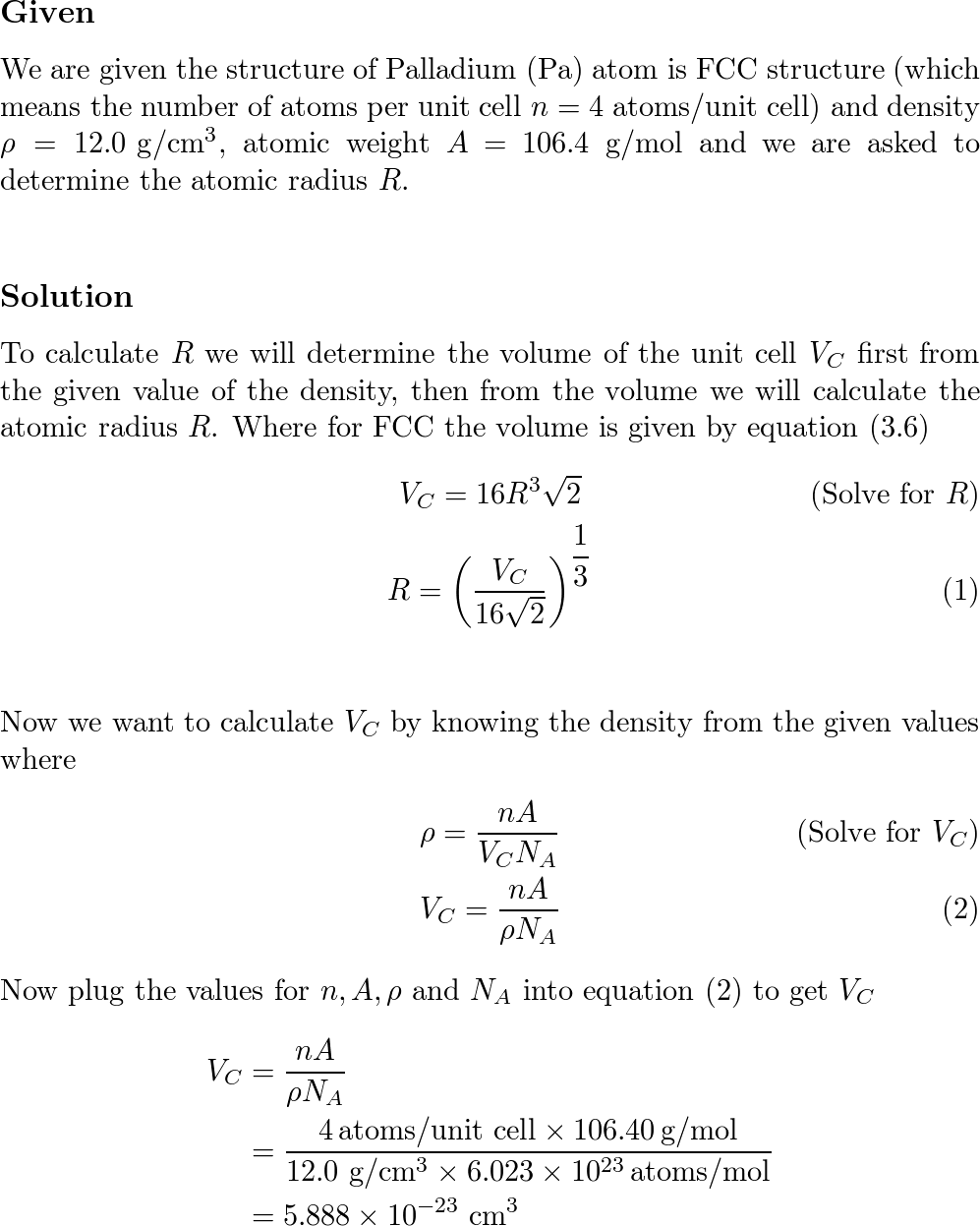
SOLVED: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.0 g/cm³. Calculate the atomic radius of palladium: (a) 138 pm, (b) 1.95 * 10⠻⠹ nm, (c) 1.95 *

OneClass: A metal crystallizes in the face-centered cubic (FCC) lattice. The density of the metal is ...

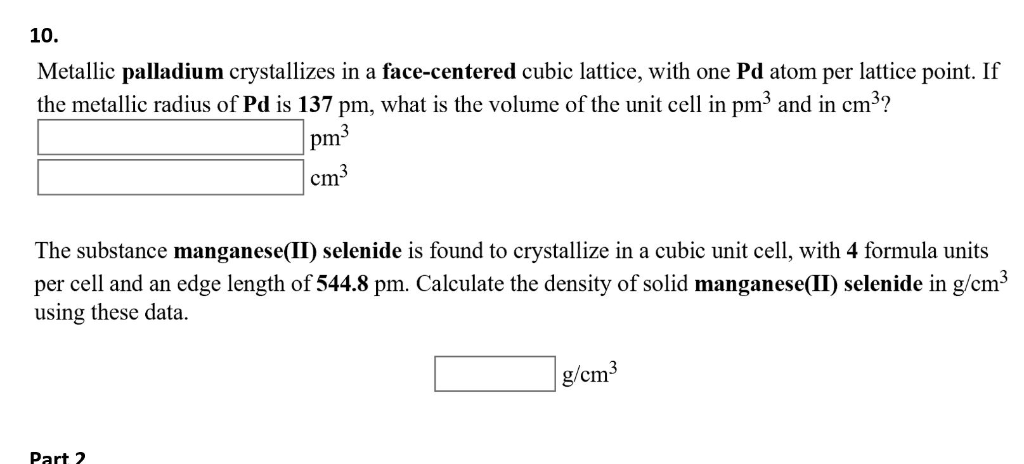
Metallic lead crystallizes in a face-centred cubic lattice, with one Pb atom per lattice point. If the metallic radius of Pb is 175 pm, what is the volume of the unit cell

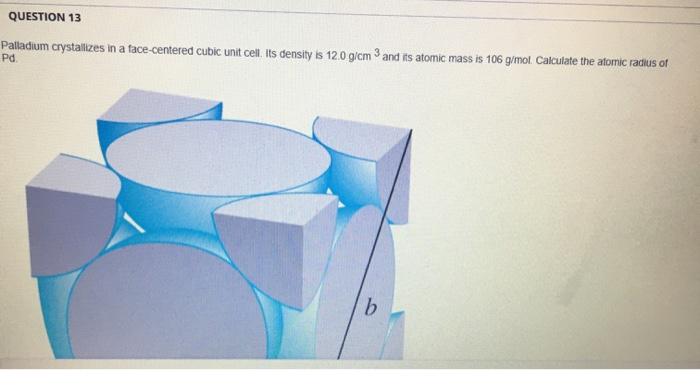
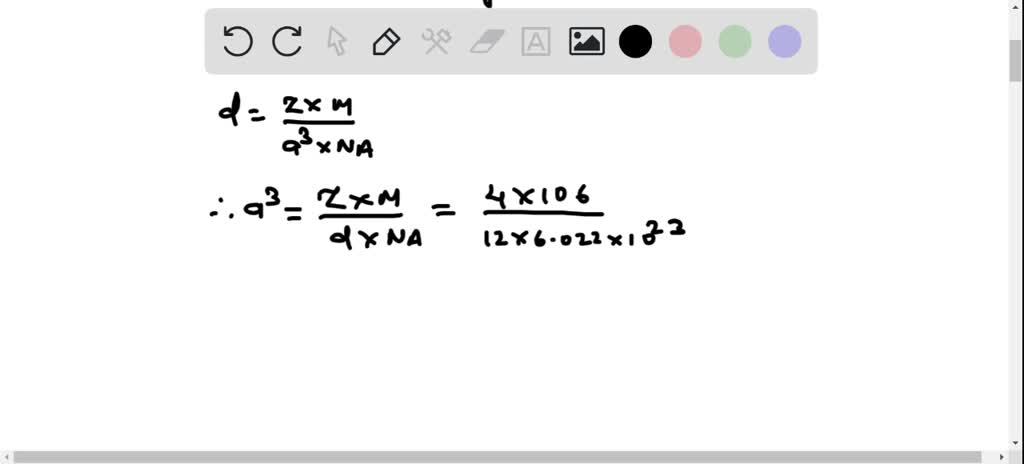
SOLVED: 1. Palladium (at. wt. = 106) crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium and its packing efficiency.

Copper has a face-centered cubic unit cell. How many atoms of Cu are present in each unit cell? | Homework.Study.com